How Many Electrons In H
A hydrogen ion is created when a hydrogen atom loses or gains an electron. A positively charged hydrogen ion (or proton) can readily combine with other particles and therefore is only seen isolated when it is in a gaseous state or a nearly particle-free space.[1] Due to its extremely loftier charge density of approximately 2×1010 times that of a sodium ion, the bare hydrogen ion cannot exist freely in solution as information technology readily hydrates, i.e., bonds rapidly.[2] The hydrogen ion is recommended by IUPAC as a full general term for all ions of hydrogen and its isotopes.[3] Depending on the charge of the ion, two unlike classes can be distinguished: positively charged ions and negatively charged ions.
Cation (positively charged) [edit]
A hydrogen atom is made up of a nucleus with charge +1, and a unmarried electron. Therefore, the simply positively charged ion possible has accuse +ane. It is noted H+.
Depending on the isotope in question, the hydrogen cation has dissimilar names:
- Hydron: general name referring to the positive ion of whatsoever hydrogen isotope (H+)
- Proton: 1H+ (i.due east. the cation of protium)
- Deuteron: 2H+, D+
- Triton: threeH+, T+
In addition, the ions produced past the reaction of these cations with h2o besides as their hydrates are chosen hydrogen ions:
- Hydronium ion: HiiiO+
- Zundel cation: H5O2 + (named for Georg Zundel)
- Eigen cation: H9O4 + (or HiiiO+ •3H2O) (named for Manfred Eigen)
Zundel cations and Eigen cations play an important role in proton diffusion according to the Grotthuss mechanism.
In connectedness with acids, "hydrogen ions" typically refers to hydrons.
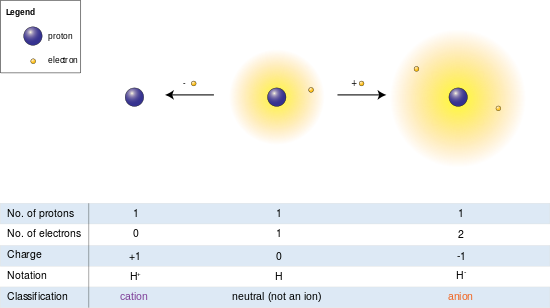
In the image at left the hydrogen cantlet (middle) contains a unmarried proton and a single electron. Removal of the electron gives a cation (left), whereas addition of an electron gives an anion (right). The hydrogen anion, with its loosely held two-electron cloud, has a larger radius than the neutral atom, which in turn is much larger than the bare proton of the cation. Hydrogen forms the simply cation that has no electrons, merely even cations that (unlike hydrogen) still retain 1 or more electrons are yet smaller than the neutral atoms or molecules from which they are derived.
Anion (negatively charged) [edit]
Hydrogen anions are formed when additional electrons are acquired:
- Hydride: full general name referring to the negative ion of any hydrogen isotope (H−)
- Protide: 1H−
- Deuteride: 2H−, D −
- Tritide: threeH−, T −
Uses [edit]
Hydrogen ions drive ATP synthase in photosynthesis. This happens when hydrogen ions get pushed across the membrane creating a high concentration inside the thylakoid membrane and a low concentration in the cytoplasm. Yet, because of osmosis, the H+ will strength itself out of the membrane through ATP synthase. Using their kinetic energy to escape, the protons volition spin the ATP synthase which in turn volition create ATP. This happens in cellular respiration likewise though the concentrated membrane volition instead be the inner membrane of the mitochondria.
Hydrogen ions concentration, measured as pH, is likewise responsible for the acidic or basic nature of a chemical compound. H2o molecules split to class H+ and hydroxide anions. This process is referred to as the self-ionization of water.
Ocean acidification [edit]
The concentration of hydrogen ions and pH are inversely proportional; in an aqueous solution, an increased concentration of hydrogen ions yields a low pH, and afterward, an acidic product. By definition, an acid is an ion or molecule that can donate a proton, and when introduced to a solution it will react with water molecules (H2O) to form a hydronium ion (H3O+), a conjugate acid of water.[iv] For simplistic reasoning, the hydrogen ion (H+) is often used to abbreviate the hydronium ion.
Ocean acidification is the direct effect of elevated concentrations of hydrogen ions and carbonate saturation from significant absorption of carbon dioxide (CO2) by the earth'southward oceans.[v] The pre-industrial state of the body of water's carbonate chemistry has been notably stable, including the balance of its pH.[6] Following the industrial revolution, anthropogenic emissions of called-for fossil fuels, cement production, and changes in land use, have increased the oceans uptake of carbon dioxide from the atmosphere past 30%.[7] In the ocean, the absorption capacity of this greenhouse gas is 59 times higher than in the atmosphere;[8] the ocean acts as the largest carbon sink on the planet, playing a significant role in climate regulation.[9] In addition to carbon fluxes, the natural procedure of carbon sequestration from the atmosphere into the deep sea is facilitated past 2 systems, the biological pump and the solubility pump. The solubility pump is a physico-chemical process that transfers COii at the air-sea interface.[10] Based on Henry's Police force, the corporeality of dissolved CO2 in an aqueous solution is directly proportional to the partial pressure of COtwo in the atmosphere.[11] To maintain equilibrium, a state of high atmospheric partial pressure of COtwo leads to an increased oceanic exchange of this gas by molecular improvidence.
In the surface waters, dissolved atmospheric carbon dioxide (COtwo(aq)) reacts with water molecules to course carbonic acid (H2COthree), a weak diprotic acid. Diprotic acids consist of two ionizable hydrogen atoms in each molecule.[12] In an aqueous solution, partial dissociation of carbonic acid releases a hydrogen proton (H+) and a bicarbonate ion (HCO3 −), and subsequently, the bicarbonate ion dissociates into an boosted hydrogen proton and a carbonate ion (COiii ii-).[13] The dissolving and dissociating of these inorganic carbon species generate an increase in the concentration of hydrogen ions and inversely lowers ambient surface bounding main pH. The carbonate buffering system governs the acidity of seawater by maintaining dissolved inorganic carbon species in chemical equilibrium.
The chemical equation consists of reactants and products that may react in either management. More reactants added to a system yield more than product production (the chemical reaction shifts to the correct) and if more product is added, boosted reactants volition course, shifting the chemical reaction to the left. Therefore, in this model, a high concentration of the beginning reactant, carbon dioxide, produces an increased amount of terminate-product (H+ and COthree 2-), thus lowering pH and creating a more than acidic solution. The natural buffering organisation of the ocean resist the alter in pH past producing more than bicarbonate ions generated by costless acid protons reacting with carbonate ions to form an alkaline grapheme.[14] Withal, increasing atmospheric CO2 concentrations may exceed the buffering capacity threshold, consequently resulting in higher rates of ocean acidification. Shifts in the ocean'southward carbonate chemistry has the potential to dispense body of water biogeochemical cycles for many elements and compounds causing profound impacts on marine ecosystems. Furthermore, the solubility of CO2 is temperature dependent; elevated surface water temperatures reduce CO2 solubility. A continual ascension in atmospheric partial pressure of COtwo could potentially convert the ocean from acting equally sink (the vertical transport of carbon to the depths of the ocean) to becoming a source (CO2 degassing from the bounding main), further increasing global temperatures.[xv]
See also [edit]
- Acid
- Protonation
- Dihydrogen cation
- Trihydrogen cation
References [edit]
- ^ "Hydrogen ion - chemistry". britannica.com . Retrieved 18 March 2018.
- ^ due to its extremely high charge density of approximately 2×x10 times that of a sodium ion
- ^ Compendium of Chemical Terminology, 2d edition McNaught, A.D. and Wilkinson, A. Blackwell Science, 1997 ISBN 0-86542-684-eight, also online Archived 2005-12-12 at the Wayback Car
- ^ OpenStax, Chemical science. OpenStax CNX. Jun 20, 2016 http://cnx.org/contents/85abf193-2bd2-4908-8563-90b8a7ac8df6@nine.311.
- ^ W.S. Broecker, T. Takahashi (1997) Neutralization of fossil fuel CO2 past marine calcium carbonate
- ^ P.Northward. Pearson, M.R. Palmer (2000) Atmospheric carbon dioxide concentrations over the past lx million years Nature, 406, pp. 695-699
- ^ C.50. Sabine, et al. (2004). The oceanic sink for anthropogenic CO2 Scientific discipline, 305 (5682), pp. 367-371
- ^ Lal R. (2008). Carbon sequestration. Philosophical transactions of the Royal Lodge of London. Series B, Biological sciences, 363(1492), 815–830. https://doi.org/x.1098/rstb.2007.2185
- ^ Ben I. Mcneil & Richard J. Matear (2007). Climate modify feedbacks on future oceanic acidification, Tellus B: Chemic and Physical Meteorology, 59:2, 191-198
- ^ Hessen, D., Ågren, G., Anderson, T., Elser, J., & De Ruiter, P. (2004). Carbon Sequestration in Ecosystems: The Part of Stoichiometry. Ecology, 85(v), 1179-1192. Retrieved November 22, 2020, from http://www.jstor.org/stable/3450161
- ^ Avishay DM, Tenny KM. Henry's Constabulary. [Updated 2020 Sep 7]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK544301/
- ^ OpenStax, Chemistry. OpenStax CNX. Jun 20, 2016 http://cnx.org/contents/85abf193-2bd2-4908-8563-90b8a7ac8df6@9.311.
- ^ OpenStax, Chemistry. OpenStax CNX. Jun 20, 2016 http://cnx.org/contents/85abf193-2bd2-4908-8563-90b8a7ac8df6@nine.311.
- ^ Middelburg, J. J., Soetaert, K., & Hagens, M. (2020). Bounding main Alkalinity, Buffering and Biogeochemical Processes. Reviews of geophysics (Washington, D.C. : 1985), 58(3), e2019RG000681. https://doi.org/10.1029/2019RG000681
- ^ Matsumoto, Chiliad. (2007). Biology-mediated temperature command on atmosphericpCO2and ocean biogeochemistry. Geophysical Inquiry Letters, 34(20). doi:10.1029/2007gl031301
How Many Electrons In H,
Source: https://en.wikipedia.org/wiki/Hydrogen_ion
Posted by: porterfrored.blogspot.com


0 Response to "How Many Electrons In H"
Post a Comment